Grasping the notion of valence electrons is essential in the field of chemistry. These electrons, located in the outermost shell of an atom, play a significant role in influencing the interactions between atoms. Oxygen, an element indispensable for life, possesses distinct properties related to its valence electrons.
Valence Electrons in Oxygen: A Closer Look
The Electronic Configuration of Oxygen
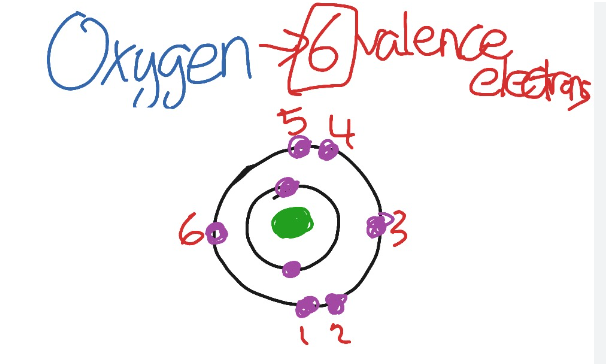
A comprehensive understanding of oxygen’s valence electrons necessitates an examination of its electronic configuration. With an atomic number of 8, oxygen possesses 8 protons and, in its neutral state, an equal number of electrons. The electronic configuration can be expressed as 1s² 2s² 2p⁴, indicating the distribution of electrons across its energy levels.
In this configuration, the first energy level, designated as 1s, accommodates 2 electrons. The second energy level consists of the 2s sublevel, which also contains 2 electrons, while the 2p sublevel within the same energy level holds 4 electrons. This arrangement is crucial for understanding the chemical properties and reactivity of oxygen.
ALSO READ : Where Did Sydney Affolter Go To High School
Valence Electrons in Oxygen
Valence electrons refer to the electrons located in the outermost energy shell of an atom. In the case of oxygen, these electrons are found within the 2s and 2p orbitals. More precisely, oxygen contains two electrons occupying the 2s orbital and four electrons residing in the 2p orbital.
Consequently, when these quantities are combined, it is determined that oxygen possesses a total of six valence electrons. This configuration plays a crucial role in the chemical behavior and bonding characteristics of oxygen in various compounds.
Significance of Valence Electrons
Valence electrons are crucial in the processes of chemical bonding and reactions. With six valence electrons, oxygen exhibits a high level of reactivity, allowing it to form bonds with a variety of other elements. Generally, oxygen seeks to acquire two additional electrons to attain a stable electronic configuration that mirrors that of the noble gas neon, which possesses eight electrons in its outermost shell.
The behavior of oxygen in chemical interactions underscores the significance of valence electrons in determining an element’s reactivity and bonding capabilities. By gaining two electrons, oxygen achieves a more stable state, which is essential for its role in various chemical processes and compounds. This tendency to form bonds is a fundamental aspect of oxygen’s chemistry, influencing its interactions with other elements in nature.
ALSO READ : Where Did Taylor Swift Stay In Chicago
Oxygen in Chemical Bonds
Oxygen typically engages in two primary types of bonding when forming compounds. The first type is covalent bonding, where oxygen shares its valence electrons with other atoms to attain a stable electron configuration. A notable example of this is found in water (H₂O), where oxygen shares electrons with two hydrogen atoms.
The second type of bonding is ionic bonding, which occurs when oxygen accepts electrons from metallic elements, resulting in the formation of anions. A clear illustration of this process can be seen in magnesium oxide (MgO), where oxygen acquires two electrons from magnesium, thereby becoming an O²⁻ ion.
Conclusion
In summary, oxygen possesses six valence electrons, which are located in its outermost energy shell. These valence electrons play a pivotal role in oxygen’s chemical behavior and bonding characteristics. Through the process of covalent bonding or ionic bonding, oxygen strives to achieve a stable electron configuration, often by acquiring two additional electrons. This tendency to form bonds is a fundamental aspect of oxygen’s chemistry, influencing its interactions with other elements in nature and its significance in various compounds and biological processes.
ALSO READ : Meet Jameliz S ,Jelly Bean Brains Real Name
FAQ’S
Is the valency of oxygen 2 or 6?
It takes or accepts 2 electrons to achieve 8 electrons in its outermost shell to complete the octet and become stable. Hence the valency of oxygen is 2.
How many valence is in O2?
Oxygen contains 6 valence electrons, as the electronic configuration of oxygen is 2,8. In ion, 2 more electron is present. So, the valence electrons in the ion are 8.
Are valence electrons always 8?
The octet rule is that an atom will be most stable when surrounded by 8 electrons in the valence shell. An atom that does not have eight electrons will bond with other atoms to have eight electrons.